1.09.2025
After the formation of the Solar System, it took a maximum of three million years for the chemical composition of the Earth's precursor to be completed. This is shown by a new study by the Institute of Geological Sciences at the University of Bern. At this time, however, there were hardly any elements necessary for life such as water or carbon compounds on the young planet. Only a later planetary collision probably brought water to Earth, paving the way for life.
Earth is so far the only known planet on which life exists – with liquid water and a stable atmosphere. However, the conditions were not conducive to life when it formed. The gas-dust cloud from which all the planets in the Solar System formed was rich in volatile elements essential for life, such as hydrogen, carbon and sulphur. However, in the inner Solar System – the part closest to the Sun, where the four rocky planets Mercury, Venus, Earth and Mars and the asteroid belt are located today – these volatile elements could hardly exist: Due to the high temperature of the Sun, they did not condense and initially remained largely in the gas phase. As these gaseous substances were not incorporated into the solid rocky materials from which the planets were formed, the early precursor of the Earth, the so-called proto-Earth, also contained very little of these vital substances. Only celestial bodies that formed further away from the Sun in cooler regions were able to incorporate these components. When and how the Earth became a life-friendly planet is still not fully understood.
In a new study, researchers from the Institute of Geological Sciences at the University of Bern have now been able to show for the first time that the chemical composition of the early Earth was complete no later than three million years after the formation of the Solar System – and in a way that initially made the emergence of life impossible. Their results, recently published in the journal Science Advances, suggest that life on Earth was only made possible by a later event. Dr. Pascal Kruttasch is first author of the study, which was part of his dissertation at the Institute of Geological Sciences and was financially supported by the Swiss National Science Foundation. Kruttasch is now an SNSF Postdoc.Mobility Fellow at Imperial College London.
Using a precise clock to measure the history of the Earth's formation
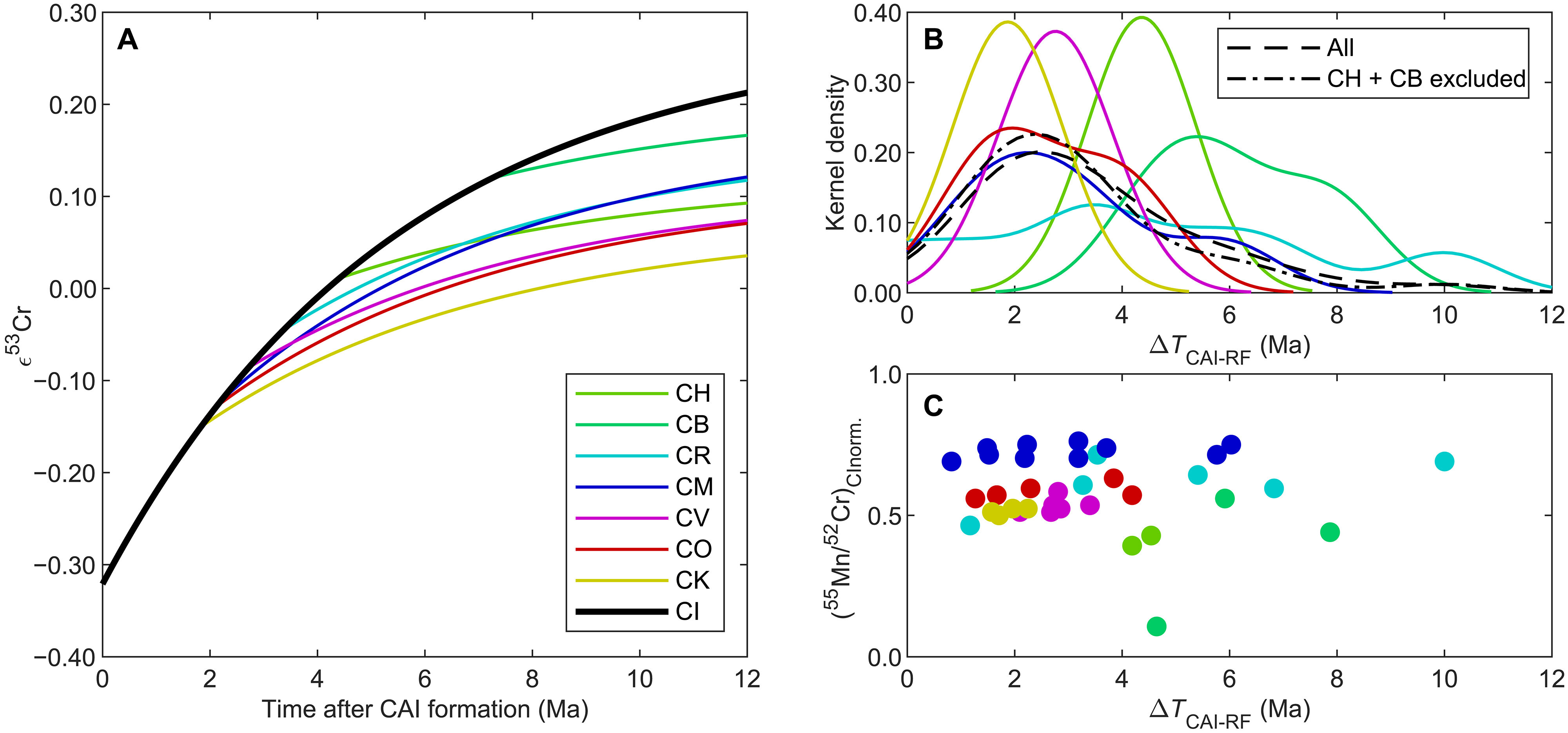
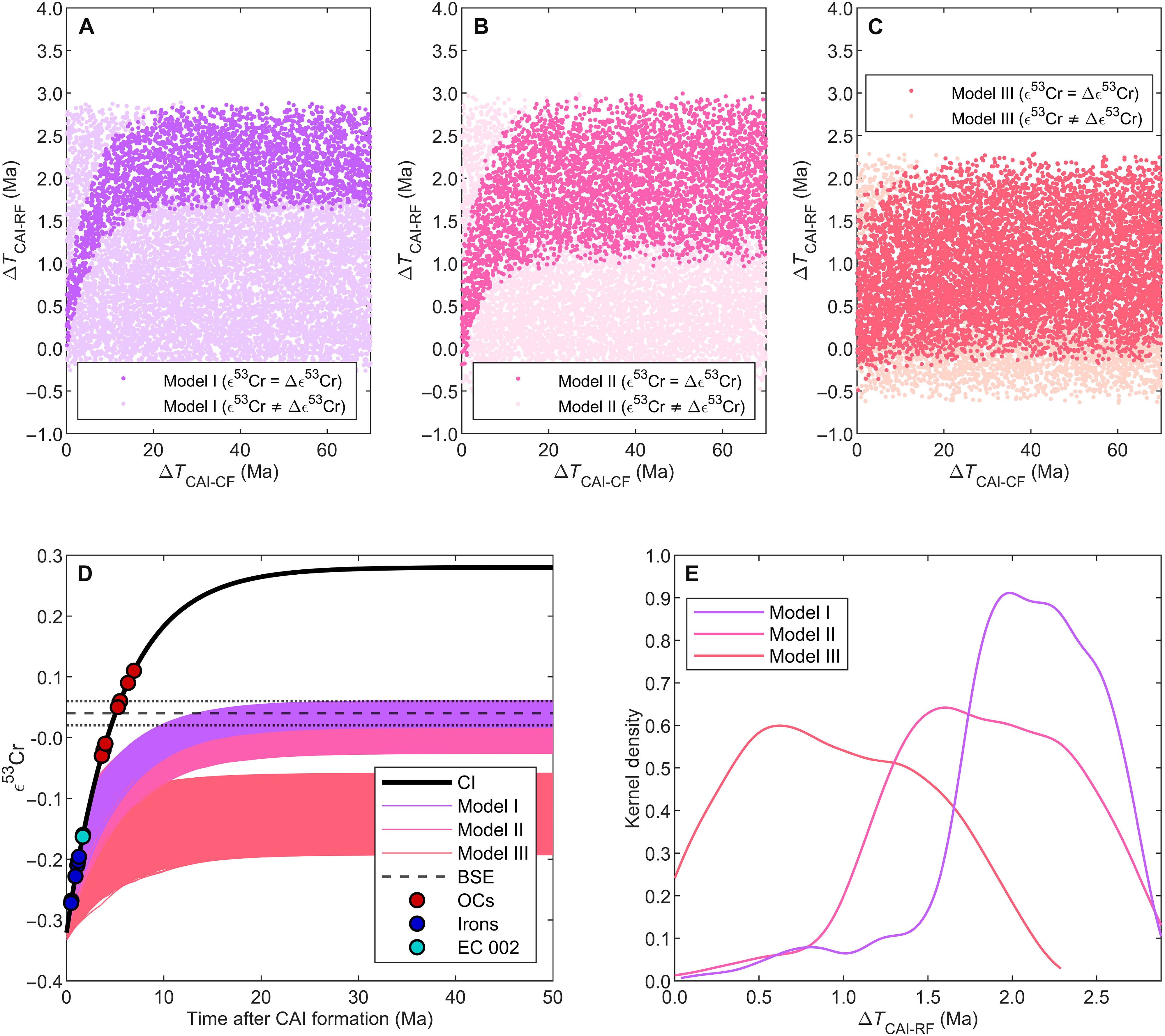
The research team used a combination of isotope and element data from meteorites and terrestrial rocks to reconstruct the process of the Earth's formation. Using model calculations, the researchers were able to narrow down in time how the chemical composition of the Earth developed in comparison to other planetary building blocks.
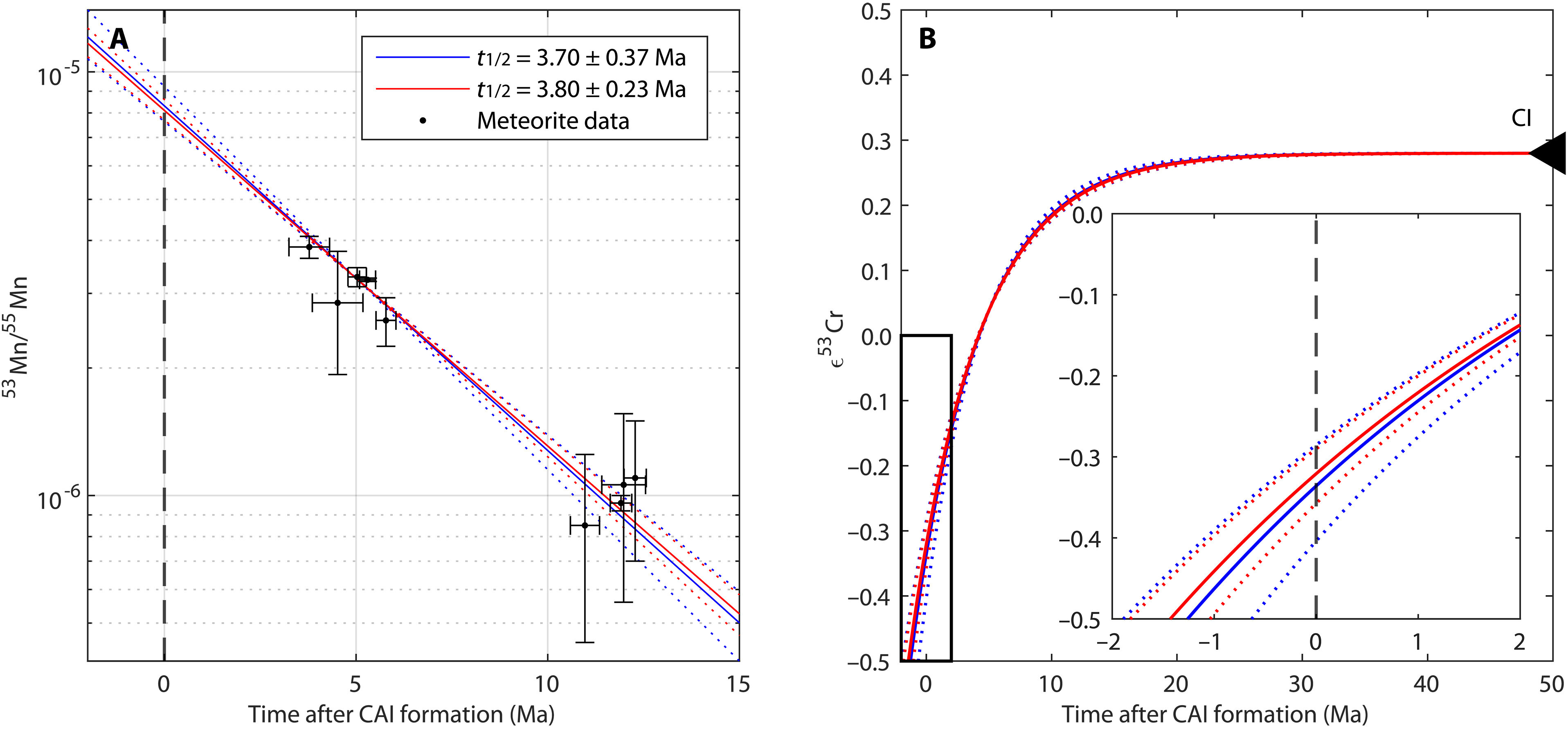
Kruttasch explains: "A high-precision time measurement system based on the radioactive decay of manganese-53 was used to determine the precise age. This isotope was present in the early Solar System and decayed to chromium-53 with a half-life of around 3.8 million years." This method allowed ages to be determined with an accuracy of less than one million years for materials that are several billion years old. "These measurements were only possible because the University of Bern has internationally recognized expertise and infrastructure for the analysis of extraterrestrial materials and is a leader in the field of isotope geochemistry," says co-author Klaus Mezger, Professor Emeritus of Geochemistry at the Institute of Geological Sciences at the University of Bern.
Life on Earth thanks to a cosmic coincidence?
Using model calculations, the research team was able to show that the chemical signature of the proto-Earth, i.e. the unique pattern of chemical substances of which it is composed, was already complete less than three million years after the formation of the Solar System. Their study thus provides empirical data on the time of formation of the original material of the young Earth. "Our Solar System formed around 4,568 million years ago. Considering that it only took up to 3 million years to determine the chemical properties of the Earth, this is surprisingly fast," says first author Kruttasch.
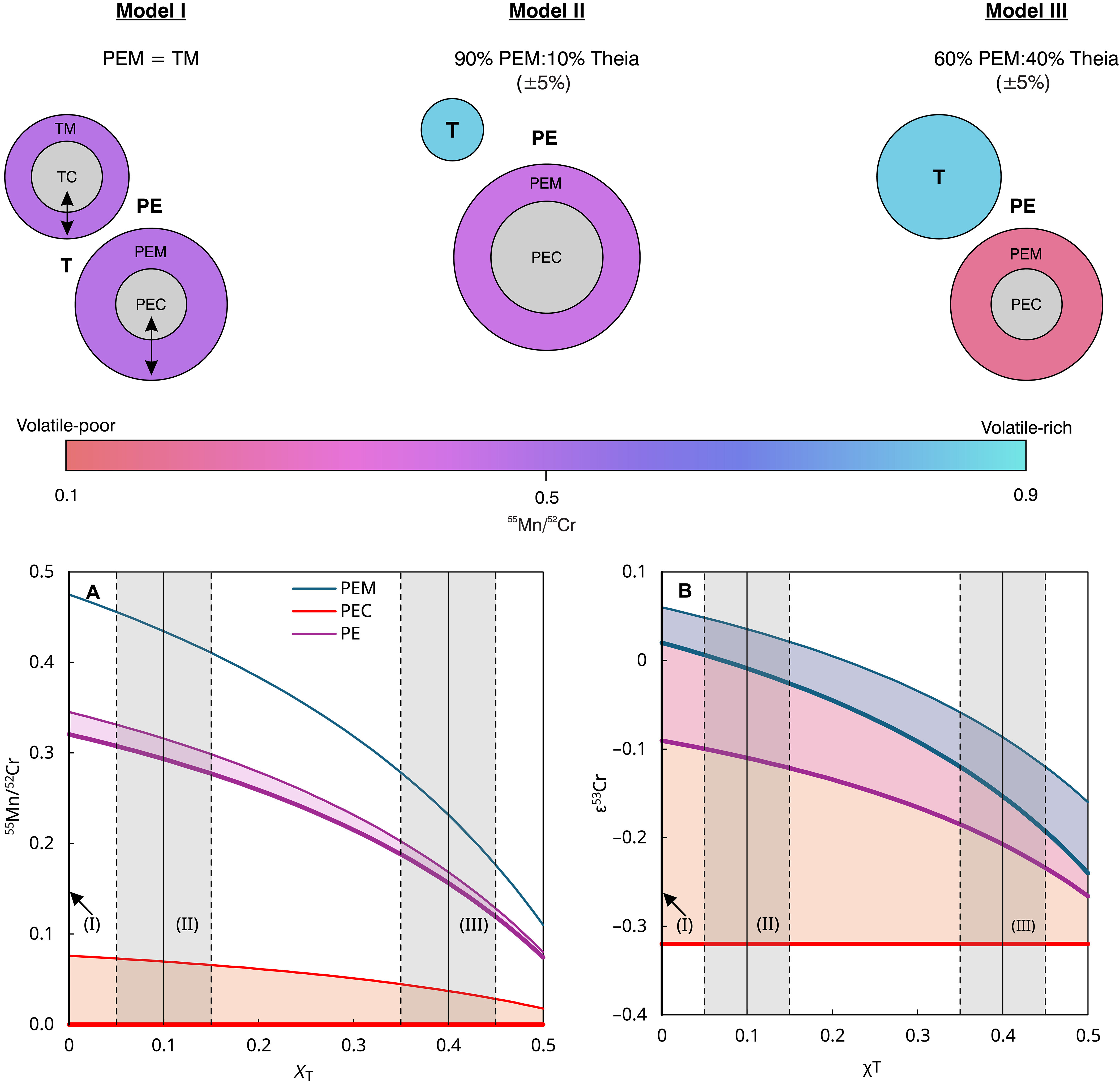
The results of the study thus support the assumption that a later collision with another planet – Theia – brought the decisive turning point and made the Earth a life-friendly planet. Theia probably formed further out in the Solar System, where volatile substances such as water accumulated. "Thanks to our results, we know that the proto-Earth was initially a dry rocky planet. It can therefore be assumed that it was only the collision with Theia that brought volatile elements to Earth and ultimately made life possible there," says Kruttasch.
Life-friendliness in the universe cannot be taken for granted
The new study contributes significantly to our understanding of the processes in the early phase of the Solar System and provides clues as to when and how planets on which life is possible can form. "The Earth does not owe its current life-friendliness to a continuous development, but probably to a chance event – the late impact of a foreign, water-rich body. This makes it clear that life-friendliness in the universe is anything but a matter of course," says Mezger.
The next step would be to investigate the collision event between proto-Earth and Theia in more detail. "So far, this collision event is insufficiently understood. Models are needed that can fully explain not only the physical properties of the Earth and Moon, but also their chemical composition and isotope signatures," concludes Kruttasch.
Quelle: University of Bern
+++
Time of proto-Earth reservoir formation and volatile element depletion from 53Mn-53Cr chronometry
Abstract