29.09.2024
Paleo-megaripples - as seen in Mars' Terra Sirenum by the High-Resolution Imaging ScienceExperiment - have consistent crest wavelengths, numerous fractures and craters, and can be partially covered by ancient lava flows.
NaNotics LLC, a biopharmaceutical company specializing in subtractive nanoparticles called NaNots, has been chosen to participate in the first-ever SPACE-H Accelerator program. This initiative, a collaboration between Starburst, NASA's Human Research Program (NASA HRP), the Translational Research Institute for Space Health (TRISH), Methuselah Foundation, and Microsoft Federal, is designed to foster innovations that address the health and performance risks associated with human spaceflight. The program's mission is "to support entrepreneurs working to advance biological and medical capabilities with the potential to minimize the health and performance risks in human spaceflight."
Space presents serious health challenges for astronauts due to its harsh environment. Extended exposure to space conditions can accelerate aging and increase the risk of diseases. Additionally, the limited space, weight, and training needed to operate Earth-based medical equipment make it difficult to deliver conventional treatments. The growing interest in space exploration, such as NASA's Artemis program aimed at establishing a permanent lunar base, underscores the need for advanced research and autonomous health systems that can safeguard astronauts' health on long missions.
"Space-H has been set up specifically to accelerate advanced health systems to support space exploration," commented Elizabeth Reynolds, Starburst's USA Managing Director. "We selected NaNotics LLC for our inaugural program because NaNots - a highly novel therapeutic platform - have the potential to address significant medical challenges associated with deep space travel."
NaNots are a new class of medicine designed to treat cancer and inflammatory diseases by adsorbing and removing disease-causing molecules from the bloodstream. For example, significant tumor suppression has been observed through the adsorption of tumor-generated immune inhibitors. The Mayo Clinic reported the results of NaNots in a humanized mouse model of triple negative breast cancer, a condition that does not respond well to traditional therapies. Additionally, NaNots can be used to prevent deadly cytokine storms by rapidly neutralizing inflammatory molecules that cause these reactions. Their simplicity makes them ideal for space missions, as they can be administered by injection and monitored using portable blood analyzers.
"We're very excited to have been selected for the first cohort of the SPACE-H program," said Lou Hawthorne, CEO of NaNotics and inventor of NaNots. "The SPACE-H program is run by some of the world's top technologists; their interest in our work speaks to the advanced nature of the NaNot platform and its potential significance for maintaining health in space as well as on Earth."
Quelle: SD
+++
NaNots for Astronauts
Lou Hawthorne, CEO, NaNotics LLC, inventor of NaNots August 30th, 2024
Contents
1. Overview
2. Space Travel & Disease
3. About NaNots
4. Diseases Treatable with NaNots
5. Why use NaNots in Space?
6. Current NaNot Development Status
7. References
Overview
Long duration spaceflight poses unique health challenges for the crew. Environmental factors can trigger
disease, and treatment options are limited by the size, weight and training required to operate medical
technology commonly used on Earth, as well as the difficulty of conducting remote medical exams by
Earthside personnel.
NaNots – under development by NaNotics, LLC, in collaboration with Mayo Clinic and Massachusetts
General Hospital (MGH) – are a new class of medicine with unique capabilities that lend themselves well
to diagnosing and treating serious diseases in deep space, with limited technology or support from Earth.
This paper outlines the challenges of treating diseases in space, and describes the capabilities of the
NaNot platform – both generally and as applied to space medicine. The paper concludes with the current
development status of the NaNot pipeline.
Space Travel & Disease
Space travel carries with it elevated risk of multiple diseases, including cancer, cardiovascular disease
(CVD), neurological disorders and accelerated aging. There are three main drivers of these disorders:
radiation (Barcellos-Hoff et al., 2015), microgravity (Dhar et al., 2021), and stress. Earth is shielded from
solar and cosmic radiation by multiple kilometers of atmosphere and a powerful magnetic field, neither of
which is practical on a spacecraft. NASA is actively studying radiation countermeasures (Crucian et al.,
2018), but for the foreseeable future spaceflight will necessarily involve increased radiation exposure, a
known trigger of cancer (Barcellos-Hoff, 2008).
Reduced or zero gravity is also known to induce a range of health problems, including “loss of muscle
mass and bone density, impaired vision, decreased kidney function, diminished neurological responses,
and a compromised immune system” (Bradbury et al., 2020).
Stress produces inflammation that mimics aging – aka “inflamm-aging” (Buchheim et al., 2019) (Young
and Sutton, 2021) – and other forms of dysregulated immune response (Crucian et al., 2015), which can
lead to arthritis, cardiovascular disease and diabetes. Prolonged isolation can also lead to stress and
associated pathologies (Ponomarev et al., 2021). And while quarantine prior to launch can reduce the risk
of introducing infectious diseases to a space crew, reduced immune function can trigger reactivation and
shedding of latent viruses (Cohrs et al., 2008) (Rooney et al., 2019).
On Earth, patients have access to a broad range of medical infrastructure, technology, pharmaceuticals,
and expertise for diagnosing and treating disease. The more such resources one attempts to include on a
space flight, the greater the cost and time required to prepare for the journey, and the greater the
logistical complexities associated with launching, accelerating, decelerating, and landing the cargo at the
destination. Compromises have to be made; for instance, rather than bringing a 5k-15k kilo MRI machine,
a 70 kilo ultrasound device – with significantly reduced imaging capability compared with MRI – would
have to do. And while there are more than 20,000 FDA-approved pharmaceuticals available on Earth, only
190 of these are routinely stocked aboard the ISS (Tatum, 2020), a total that doesn’t include drugs
required for treating most serious diseases such as cancer (Kast et al., 2017).
The bottom line is that astronauts have both elevated risk of disease and very limited ability to carry with
them the technology and personnel needed to treat the most serious diseases they might develop. Better
treatment options are needed to safeguard astronaut health on long-duration spaceflights.
About NaNots
NaNots invert the drug paradigm of the last 2000 years: Rather than adding molecules to the body,
NaNots deplete from blood specific molecular drivers of disease. The vast majority of non-genetic
diseases are driven or enabled by one or more soluble (dissolved) factors in blood, and depletion of these
factors can be as effective or even more so than current pharmaceuticals, without their toxicity.
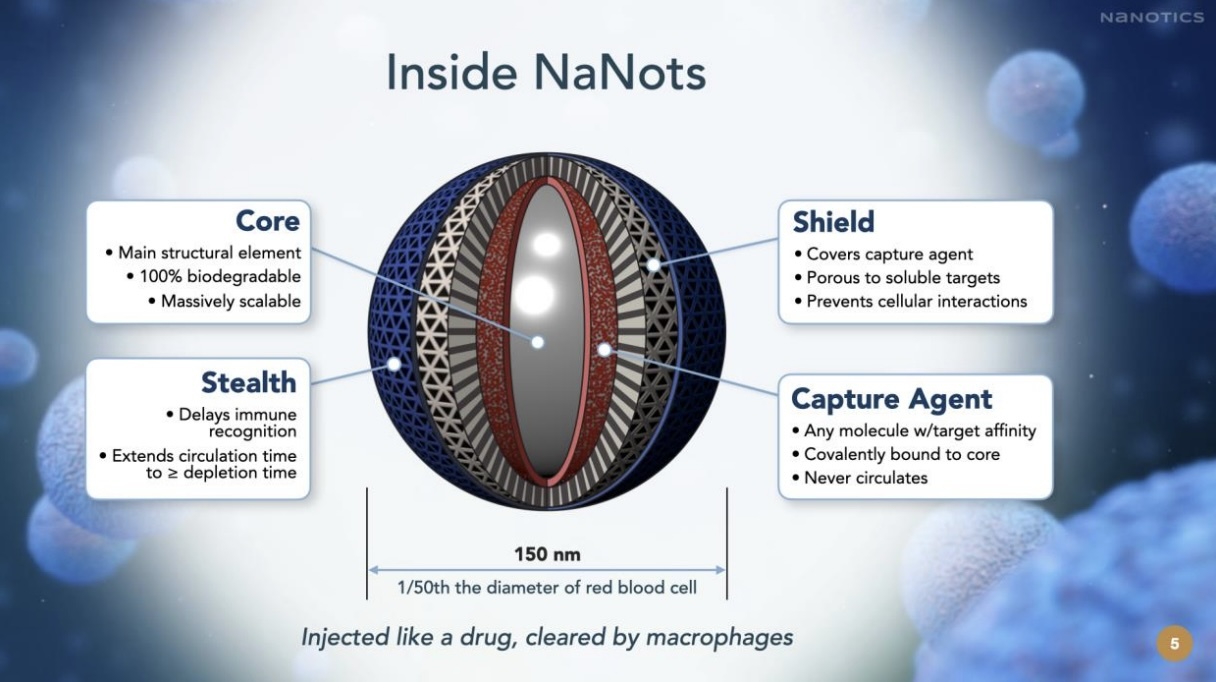
NaNots are injectable absorptive nanoparticles designed to capture specific pathogenic soluble targets
from circulation. NaNots contain capture agents specific to the target they are designed to deplete,
beneath a porous shield, which covers the capture agents and makes the NaNots specific for soluble
targets and inert relative to all cell surface proteins. Many pathogenic molecules in blood have a
biochemically identical form on a cell surface engaged in a vital process. Distinguishing between these
target forms is beyond the capability of drugs and represents a significant therapeutic advance.
Following target capture, NaNots are cleared via phagocytosis (engulfment) by liver-resident
macrophages, which break down the NaNots and their captured targets into small molecules, which are
then excreted.
NaNots deplete targets from circulation selectively, deeply and very rapidly; pre-clinical studies have
demonstrated that NaNots typically deplete their targets by greater than 95% in less than 5 minutes post-
injection. Depletion of a pathogenic target from circulation creates a powerful diffusion sink that induces
rapid target diffusion out from any localized concentrations, thereby inhibiting local disease processes.
Diseases Treatable with NaNots
The vast majority of non-genetic diseases fit into one of two categories: Inflammatory Diseases and
Inhibitory Diseases:
1. Inflammatory Diseases are driven by inflammatory cytokines and range from common chronic
diseases like arthritis and psoriasis, to potentially deadly acute inflammatory disorders such as
myocardial infarction, stroke and sepsis (Kany, Vollrath and Relja, 2019). There are also many
diseases that are newly understood to have an inflammatory basis, such as atherosclerosis (Wolf
and Ley, 2019) and type 2 diabetes (Tsalamandris et al., 2019). Diseases that look very different at
the tissue or organ level are often driven by the same inflammatory cytokines;
2. Inhibitory Diseases are characterized by abnormal tissue that the immune system allows to persist
because the tissue secretes potent immune inhibitors. An expanding body of data suggests that
most if not all tumor-forming cancers rely on these immune inhibitors for their survival (Wang et
al., 2022), and that senescent cells may as well (Coppé et al., 2010). As with inflammatory
diseases, inhibitory diseases that look very different at a tissue level – such as different types of
cancer – often rely on a relatively small set of immune inhibitors, common across cancer types.
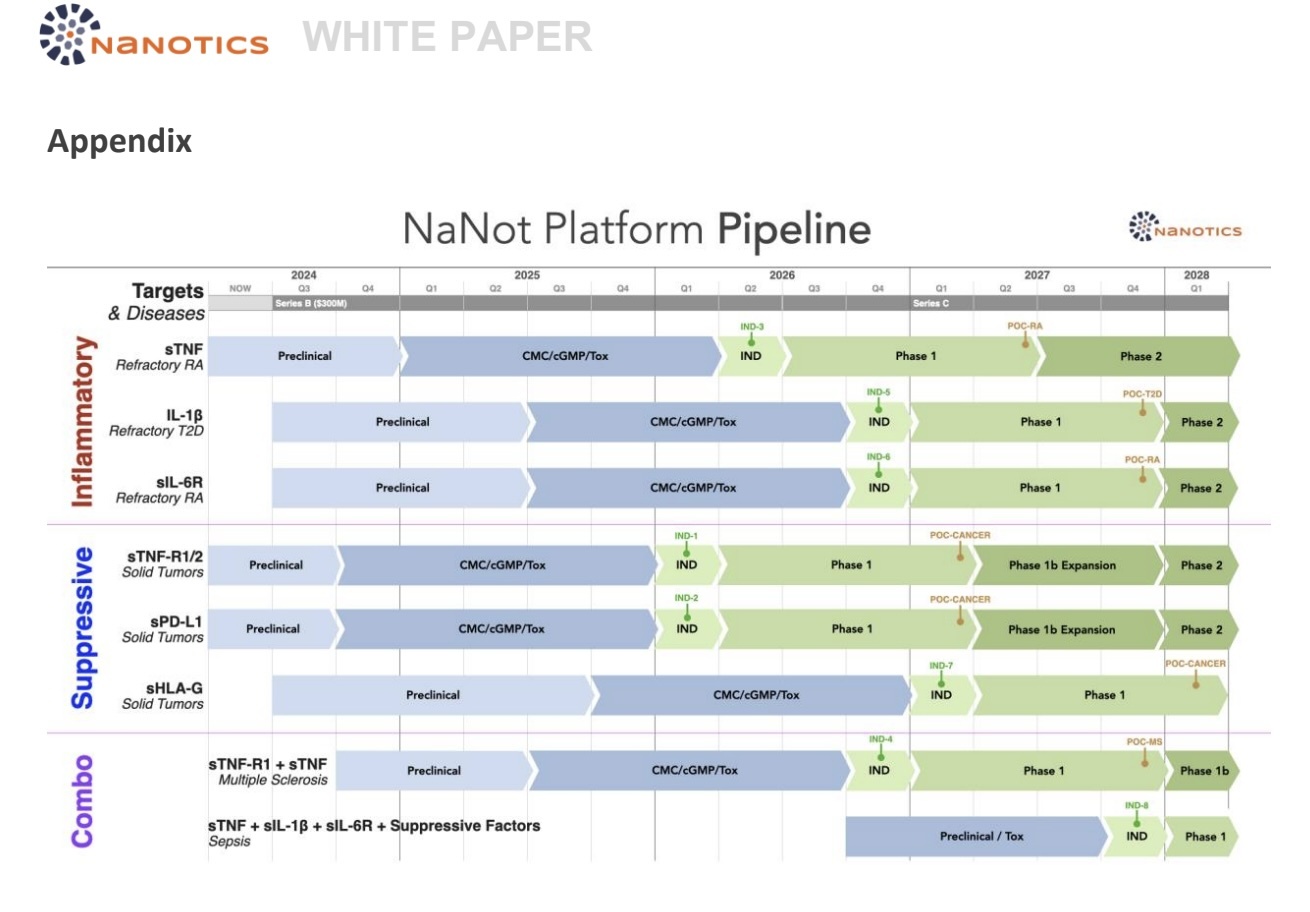
NaNotics LLC is developing an initial “toolkit” of seven NaNots, three against soluble inflammatory
molecules and four against soluble inhibitory molecules, which collectively drive a broad range of chronic
and acute diseases with significant unmet need on Earth, and which would be challenging to treat in
space using conventional drugs and devices.
See the Appendix for the planned pipeline of seven NaNots and the diseases they’ll be used to treat.
Why use NaNots in Space?
NaNots are a powerful new modality for treating disease on Earth, and they offer the following unique
advantages in space:
1. Direct disease relevance. NaNotics LLC development priorities on Earth align with disease
concerns of long-duration space flight: cancer, inflammation and associated pathologies;
2. Simple supporting tech. Target identification and quantification – which in turn guides NaNot
dosing – only requires simple lateral flow immunoassays (LFIs), which work by capillary action, not
gravity (Kolliopoulos and Kumar, 2021). NaNot administration is by simple IV injection, easily
mastered by non-medical personnel (as every heroin addict knows);
3. Proximal endpoints. Unlike drugs, NaNots have a verifiable proximal endpoint. NaNot targets exist
in blood prior to administration; the proximal endpoint of NaNot treatment is depletion of these
targets, which is quantifiable before and after treatment using LFIs. Secondary clinical endpoints
depend on various factors, but the ability to verify almost immediately that a given NaNot
treatment did what it was designed to do offers a huge advantage over conventional therapeutics;
4. Ease of data sharing. LFI output indicating target concentrations pre- and post-NaNot treatment
are simple numeric values – picograms of target per milliliter (pg/mL) of blood – and thus sharing
these values with Ground Control involves ultra-compact data transmissions;
5. Automation potential. Multiple microfluidic platforms exist – and are evolving rapidly – for
automated ID / quantification of soluble factors in blood (Sachdeva, Davis and Saha, 2021). Output
from these systems can be transmitted to Ground Control in real time, and can drive syringe
loading of NaNot doses personalized for individual astronauts. These systems further reduce the
technical processes and calculations that need to be performed by spacecraft personnel.
Current NaNot Development Status
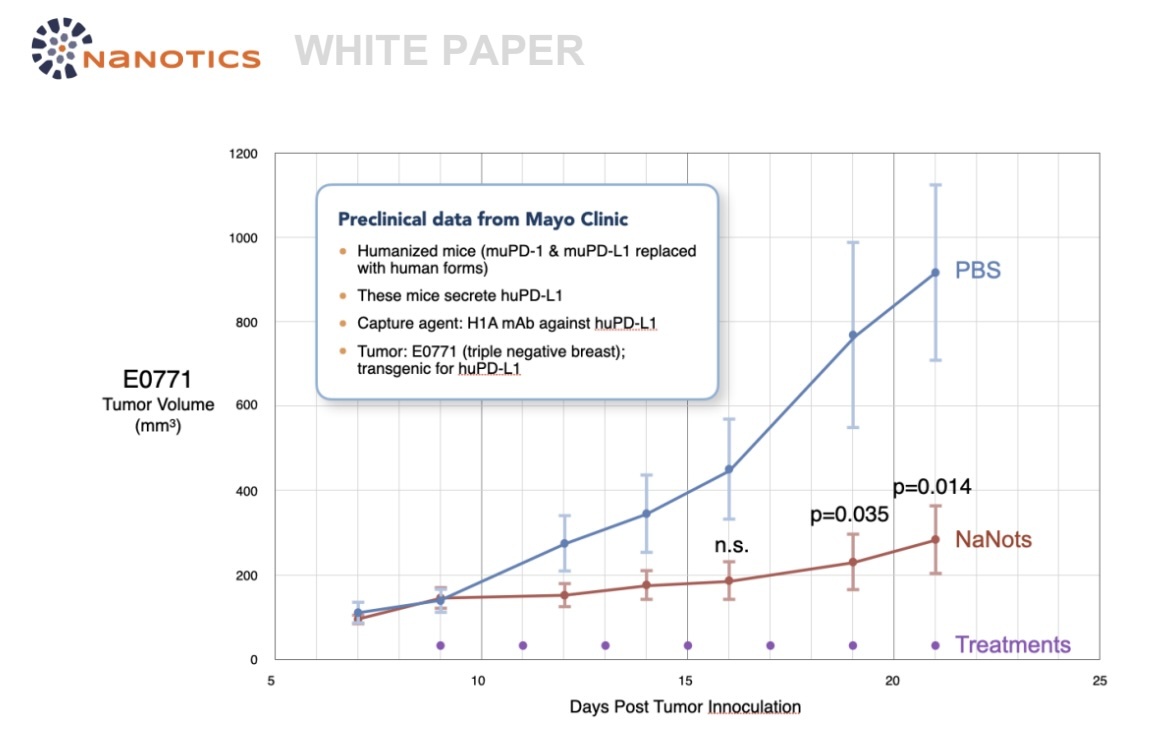
NaNots are currently in late-stage preclinical development. In November, 2022, Mayo Clinic, in
collaboration with NaNotics, tested NaNots against sPD-L1, a tumor-generated immune inhibitor (also
involved in age-related decline of immune competence), in a humanized mouse model of triple-negative
breast cancer, which responds poorly to checkpoint inhibitors in both animals and humans. Tumor
suppression was very significant, even leading to remission in some mice. These data were presented at
The Society for Immunotherapy of Cancer (SITC) in November, 2023, and are now being elaborated for
top-tier journal submission in late 2024.
The following graph summarizes tumor suppression in mice receiving NaNots against sPD-L1 versus
control mice that received PBS (saline):
NaNotics is also collaborating with MGH in Boston, under P.I. Keith Flaherty M.D., who co-founded both
Loxo Oncology ($8B exit, 2019) and Scorpion Therapeutics ($270m raised to date). Dr. Flaherty will be
testing NaNots against sTNF-Rs, another type of tumor-generated immune inhibitor (also involved in the
defense of senescent cells). Dr. Flaherty is available to speak with prospective partners about the
significance of the NaNot platform in oncology (he has no financial interest in the company).
The NaNotics lead oncology programs targeting each of the above tumor-generated immune inhibitors
are expected to progress to human studies in 2026. NaNots have shown no toxicity to date in rodent
studies, even at 100x the planned dose in humans, and are therefore expected to have a superior safety
profile to chemotherapy, checkpoint inhibitors, and other conventional therapeutics.
NaNotics is also in discussions with multiple top tier research institutions focused on sepsis, for which the
NaNot platform is uniquely well-suited. Sepsis kills more people than all forms of cancer combined
(Guzman, 2020) and is the #1 cause of death in hospital throughout most of the world (Sepsis Accounts
for 1 in 5 Deaths, Leading Cause of Death in Hospitals, 2020). NaNotics has already successfully tested a
NaNot against TNF-α, a key inflammatory cytokine involved in sepsis and other inflammatory diseases. A
company white paper on development of NaNot regimens for treating sepsis is available under NDA.
NaNotics has a large and rapidly growing IP portfolio: 38 granted patents covering broad claims for
compositions and methods, with protection in the US, Europe, Asia, Australia, Israel and elsewhere.
NaNotics IP is managed by company CTO, John Dodgson, Ph.D., a physical chemist who also oversees
NaNot engineering. The NaNotics CDO/COO is Curtis Ruegg, Ph.D., a world-class designer of drugs and
nanomedicines, who is managing the company’s manufacturing and regulatory approval processes.
Summary of Conclusions
• Long duration spaceflight poses unique health challenges for the crew, especially cancer and
inflammation leading to a range of serious pathologies.
• NaNots are a novel platform technology capable of depleting soluble molecules that drive
inflammatory diseases or enable immune evasion by cancers.
• NaNots offer unique advantages over conventional medical approaches for treating disease in
space, including low required technology infrastructure, simplicity of administration for non-
medical crew personnel, proximal endpoints, ease of data sharing and potential for automation.
• NaNots have already demonstrated dramatic preclinical efficacy in humanized mice, as well as ultra
low toxicity, and are scheduled for initial human studies within 18 months. A suite of NaNots
against various targets could potentially be approved before the first long-duration spaceflights.
Quelle: NANOTICS
